Omicron boosters: Is salesmanship trumping science?
Moderna headquarters in Cambridge, Mass.
Last month, the Food and Drug Administration authorized Omicron-specific vaccines (with Moderna’s the best known), accompanied by breathless science-by-press release and a media blitz. Just days after the FDA’s move, the Centers for Disease Control and Prevention followed, recommending updated boosters for anyone age 12 and up who had received at least two doses of the original COVID-19 vaccines. The message to a nation still struggling with the COVID pandemic: The cavalry — in the form of a shot — is coming over the hill.
But for those familiar with the business tactics of the pharmaceutical industry, that exuberant messaging — combined with the lack of completed studies — has caused considerable heartburn and raised an array of unanswered concerns.
The updated shots easily clear the “safe and effective” bar for government authorization. But in the real world, are the Omicron-specific vaccines significantly more protective — and in what ways — than the original COVID vaccines so many have already taken? If so, who would benefit most from the new shots? Since the federal government is purchasing these new vaccines — and many of the original, already purchased vaccines may never find their way into taxpayers’ arms — is the $3.2 billion price tag worth the unclear benefit? Especially when these funds had to be pulled from other covid response efforts, like testing and treatment.
Several members of the CDC advisory committee that voted 13-1 for the recommendation voiced similar questions and concerns, one saying she only “reluctantly” voted in the affirmative.
Some said they set aside their desire for more information and better data and voted yes out of fear of a potential winter COVID surge. They expressed hope that the new vaccines — or at least the vaccination campaign that would accompany their rollout — would put a dent in the number of future cases, hospitalizations, and deaths.
That calculus is, perhaps, understandable at a time when an average of more than 300 Americans are dying of COVID each day.
But it leaves front-line health care providers in the impossible position of trying to advise individual patients whether and when to take the hot new vaccines without complete data and in the face of marketing hype.
Don’t get us wrong. We’re grateful and amazed that Pfizer-BioNTech and Moderna (with assists from the National Institutes of Health and Operation Warp Speed) developed an effective vaccine in record time, freeing the nation from the deadliest phase of the covid pandemic, when thousands were dying each day. The pandemic isn’t over, but the vaccines are largely credited for enabling most of America to return to a semblance of normalcy. We’re both up-to-date with our covid vaccinations and don’t understand why anyone would choose not to be, playing Russian roulette with their health.
But as society moves into the next phase of the pandemic, the pharmaceutical industry may be moving into more familiar territory: developing products that may be a smidgen better than what came before, selling — sometimes overselling — their increased effectiveness in the absence of adequate controlled studies or published data, advertising them as desirable for all when only some stand to benefit significantly, and in all likelihood raising the price later.
This last point is concerning because the government no longer has funds to purchase COVID vaccines after this autumn. Funding to cover the provider fees for vaccinations and community outreach to those who would most benefit from vaccination has already run out. So updated boosters now and in the future will likely go to the “worried well” who have good insurance rather than to those at highest risk for infection and progression to severe disease.
The FDA’s mandated task is merely to determine whether a new drug is safe and effective. However, the FDA could have requested more clinical vaccine effectiveness data from Pfizer and Moderna before authorizing their updated omicron BA.5 boosters.
Yet the FDA cannot weigh in on important follow-up questions: How much more effective are the updated boosters than vaccines already on the market? In which populations? And what increase in effectiveness is enough to merit an increase in price (a so-called cost-benefit analysis)? Other countries, such as the United Kingdom, perform such an analysis before allowing new medicines onto the market, to negotiate a fair national price.
The updated booster vaccine formulations are identical to the original covid vaccines except for a tweak in the mRNA code to match the omicron BA.5 virus. Studies by Pfizer showed that its updated Omicron BA.1 booster provides a 1.56 times higher increase in neutralizing antibody titers against the BA.1 virus as compared with a booster using its original vaccine. Moderna’s studies of its updated Omicron BA.1 booster demonstrated very similar results. However, others predict that a 1.5 times higher antibody titer would yield only slight improvement in vaccine effectiveness against symptomatic illness and severe disease, with a bump of about 5 percent and 1 percent, respectively. Pfizer and Moderna are just starting to study their updated Omicron BA.5 boosters in human trials.
Though the studies of the updated Omicron BA.5 boosters were conducted only in mice, the agency’s authorization is in line with precedent: The FDA clears updated flu shots for new strains each year without demanding human testing. But with flu vaccines, scientists have decades of experience and a better understanding of how increases in neutralizing antibody titers correlate with improvements in vaccine effectiveness. That’s not the case with COVID vaccines. And if mouse data were a good predictor of clinical effectiveness, we’d have an HIV vaccine by now.
As population immunity builds up through vaccination and infection, it’s unclear whether additional vaccine boosters, updated or not, would benefit all ages equally. In 2022, the U.S. has seen COVID-hospitalization rates among people 65 and older increase relative to younger age groups. And while COVID vaccine boosters seem to be cost-effective in the elderly, they may not be in younger populations. The CDC’s Advisory Committee on Immunization Practices considered limiting the updated boosters to people 50 and up, but eventually decided that doing so would be too complicated.
Unfortunately, history shows that — as with other pharmaceutical products — once a vaccine arrives and is accompanied by marketing, salesmanship trumps science: Many people with money and insurance will demand it whether data ultimately proves it is necessary for them individually or not.
We are all likely to encounter the SARS-CoV-2 virus again and again, and the virus will continue to mutate, giving rise to new variants year after year. In a country where significant portions of at-risk populations remain unvaccinated and unboosted, the fear of a winter surge is legitimate.
But will the widespread adoption of a vaccine — in this case yearly updated COVID boosters — end up enhancing protection for those who really need it or just enhance drugmakers’ profits? And will it be money well spent?
The federal government has been paying a negotiated price of $15 to $19.50 a dose of mRNA vaccine under a purchasing agreement signed during the height of the pandemic. When those government agreements lapse, analysts expect the price to triple or quadruple, and perhaps even more for updated yearly COVID boosters, which Moderna’s CEO said would evolve “like an iPhone.” To deploy these shots and these dollars wisely, a lot less hype and a lot more information might help.
Elisabeth Rosenthal (erosenthal@kff.org, @rosenthalhealth) and Céline Gounder (cgounder@kff.org) are Kaiser Health News journalists.
Liz Szabo: Looking at the Interplay of Omicron, reinfections and long COVID
“I suspect there will be millions of people who acquire long COVID after Omicron infection.”
— Akiko Iwasaki, a professor of immunobiology at Yale University.
The latest COVID-19 surge, caused by a shifting mix of quickly evolving omicron subvariants, appears to be waning, with cases and hospitalizations beginning to fall.
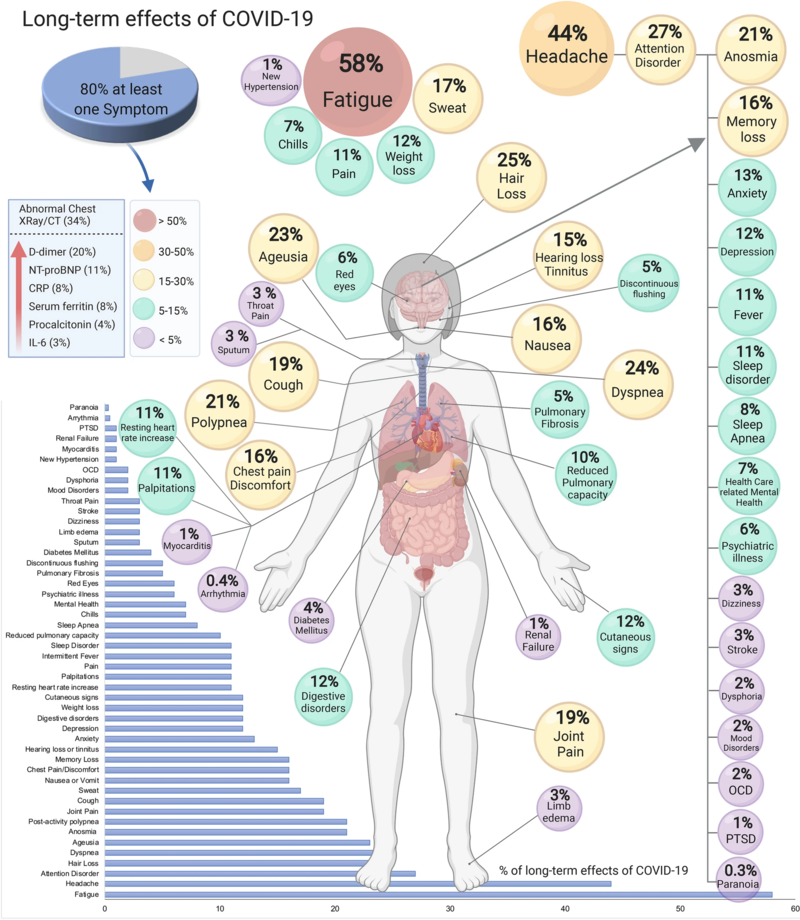
Like past COVID waves, this one will leave a lingering imprint in the form of long COVID, an ill-defined catchall term for a set of symptoms that can include debilitating fatigue, difficulty breathing, chest pain, and brain fog.
Although omicron infections are proving milder overall than those caused by last summer’s Delta variant, omicron has also proved capable of triggering long-term symptoms and organ damage. But whether omicron causes long covid symptoms as often — and as severe — as previous variants is a matter of heated study.
Michael Osterholm, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, is among the researchers who say the far greater number of Omicron infections compared with earlier variants signals the need to prepare for a significant boost in people with long covid. The U.S. has recorded nearly 38 million COVID infections so far this year, as Omicron has blanketed the nation. That’s about 40 percent of all infections reported since the start of the pandemic, according to the Johns Hopkins University Coronavirus Research Center.
Long COVID “is a parallel pandemic that most people aren’t even thinking about,” said Akiko Iwasaki, a professor of immunobiology at Yale University. “I suspect there will be millions of people who acquire long COVID after Omicron infection.”
Scientists have just begun to compare variants head to head, with varying results. While one recent study in The Lancet suggests that omicron is less likely to cause long COVID, another found the same rate of neurological problems after Omicron and Delta infections.
Estimates of the proportion of patients affected by long covid also vary, from 4 percent to 5 percent in triple-vaccinated adults to as many as 50 percent among the unvaccinated, based on differences in the populations studied. One reason for that broad range is that long COVID has been defined in widely varying ways in different studies, ranging from self-reported fogginess for a few months after infection to a dangerously impaired inability to regulate pulse and blood pressure that may last years.
Even at the low end of those estimates, the sheer number of omicron infections this year would swell long-covid caseloads. “That’s exactly what we did find in the U.K.,” said Claire Steves, a professor of aging and health at King’s College in London and author of the Lancet study, which found patients have been 24 to 50 percent less likely to develop long COVID during the Omicron wave than during the delta wave. “Even though the risk of long COVID is lower, because so many people have caught Omicron, the absolute numbers with long covid went up,” Steves said.
A recent study analyzing a patient database from the Veterans Health Administration found that reinfections dramatically increased the risk of serious health issues, even in people with mild symptoms. The study of more than 5.4 million V.A. patients, including more than 560,000 women, found that people reinfected with covid were twice as likely to die or have a heart attack as people infected only once. And they were far more likely to experience health problems of all kinds as of six months later, including trouble with their lungs, kidneys, and digestive system.
“We’re not saying a second infection is going to feel worse; we’re saying it adds to your risk,” said Dr. Ziyad Al-Aly, chief of research and education service at the Veterans Affairs St. Louis Health Care System.
Researchers say the study, published online but not yet peer-reviewed, should be interpreted with caution. Some noted that VA patients have unique characteristics, and tend to be older men with high rates of chronic conditions that increase the risks for long covid. They warned that the study’s findings cannot be extrapolated to the general population, which is younger and healthier overall.
“We need to validate these findings with other studies,” said Dr. Harlan Krumholz, director of the Yale New Haven Hospital Center for Outcomes Research and Evaluation. Still, he added, the V.A. study has some “disturbing implications.”
With an estimated 82 percent of Americans having been infected at least once with the coronavirus as of mid-July, most new cases now are reinfections, said Justin Lessler, a professor of epidemiology at the University of North Carolina Gillings School of Global Public Health.
Of course, people’s risk of reinfection depends not just on their immune system, but also on the precautions they’re taking, such as masking, getting booster shots, and avoiding crowds.
After her second COVID-19 infection, Tee Hundley, a Jersey City, N.J., \ salon owner, says her lungs seemed damaged: “I felt like I was breathing through a straw.” More than a year later, the tightness in her chest remains. “I feel like that’s something that will always be left over,” Hundley says. “You may not feel terrible, but inside of your body there is a war going on.”
After her second infection, she returned to work as a cosmetologist at her salon but struggled with illness and shortness of breath for the next eight months, often feeling like she was “breathing through a straw.”
She was exhausted, and sometimes slow to find her words. While waxing a client’s eyebrows, “I would literally forget which eyebrow I was waxing,” Hundley said. “My brain was so slow.”
When she got a breakthrough infection in July, her symptoms were short-lived and milder: cough, runny nose, and fatigue. But the tightness in her chest remains.
“I feel like that’s something that will always be left over,” said Hundley, who warns friends with covid not to overexert. “You may not feel terrible, but inside of your body there is a war going on.”
Although each Omicron subvariant has different mutations, they’re similar enough that people infected with one, such as BA.2, have relatively good protection against newer versions of omicron, such as BA.5. People sickened by earlier variants are far more vulnerable to BA.5.
Several studies have found that vaccination reduces the risk of long COVID. But the measure of that protection varies by study, from as little as a 15 percent reduction in risk to a more than 50 percent decrease. A study published in July found the risk of long COVID dropped with each dose people received.
For now, the only surefire way to prevent long covid is to avoid getting sick. That’s no easy task as the virus mutates and Americans have largely stopped masking in public places. Current vaccines are great at preventing severe illness but do not prevent the virus from jumping from one person to the next. Scientists are working on next-generation vaccines — “variant-proof” shots that would work on any version of the virus, as well as nasal sprays that might actually prevent spread. If they succeed, that could dramatically curb new cases of long COVIDF.
“We need vaccines that reduce transmission,” Al-Aly said. “We need them yesterday.”
Liz Szabo is a Kaiser Health News correspondent.